Search for a Cure


BioMarker Strategies Snappath® 1000
SnapPath® is an innovative IVD instrument for live cell processing. HS Design’s expertise was involved in Design Research, Industrial Design, Systems Engineering, User Interface Design, Mechanical Engineering, Prototyping and ultimately Formative Verification and submitted to the FDA under the 510k de novo process.

Brand
The evolution of “personalized medicine” is changing the way oncologists diagnose and treat cancer. Over the past few years, there has been a clear shift in the oncology pipeline away from traditional cytotoxic drugs to more molecularly targeted agents. Similarly, there has been a shift away from simple, single-target drugs towards inhibition of complex signaling pathways. In each case, the need for more complex functional biomarkers has emerged. SnapPath® not only provides a means for preserving functional information within a cell, it more importantly enables the generation of PathMap® profiles.
Brand
The evolution of “personalized medicine” is changing the way oncologists diagnose and treat cancer. Over the past few years, there has been a clear shift in the oncology pipeline away from traditional cytotoxic drugs to more molecularly targeted agents. Similarly, there has been a shift away from simple, single-target drugs towards inhibition of complex signaling pathways. In each case, the need for more complex functional biomarkers has emerged. SnapPath® not only provides a means for preserving functional information within a cell, it more importantly enables the generation of PathMap® profiles.

Product
The SnapPath® biomarker testing system is an automated live tumor cell processing platform that enables next-generation predictive tests — known as PathMap® profiles — to guide targeted drug therapy selection for individual cancer patients. The team sought the input of direct Users to interpret their perceptions of the SnapPath® platform and the possible integration into their lab environment or workflow.

Product
The SnapPath® biomarker testing system is an automated live tumor cell processing platform that enables next-generation predictive tests — known as PathMap® profiles — to guide targeted drug therapy selection for individual cancer patients. The team sought the input of direct Users to interpret their perceptions of the SnapPath® platform and the possible integration into their lab environment or workflow.

Creating An Experience
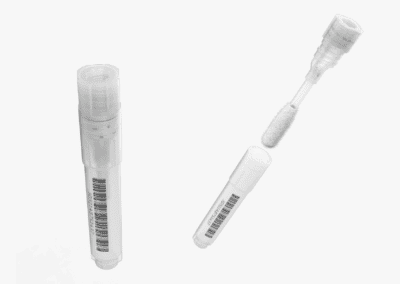
SnapPath® is a system that comprises an Instrument and a Cartridge. The Instrument is to be a Pathology-based device that is fully integrated and interfaces directly with a Cartridge preloaded with reagents and disposables. The platform processes IVD biopsy specimens procured from FNA procedures but has potential to include surgical and core samples to date. Molecular testing of solid tumors has relied primarily on the testing of dead formalin-fixed samples. The SnapPath® platform uniquely enables the ex vivo induction of functional biomarkers to show how a patient’s live tumor cells respond to pathway stimulants (such as growth factors) and inhibitors (therapeutic drugs).
Creating An Experience
SnapPath® is a system that comprises an Instrument and a Cartridge. The Instrument is to be a Pathology-based device that is fully integrated and interfaces directly with a Cartridge preloaded with reagents and disposables. The platform processes IVD biopsy specimens procured from FNA procedures but has potential to include surgical and core samples to date. Molecular testing of solid tumors has relied primarily on the testing of dead formalin-fixed samples. The SnapPath® platform uniquely enables the ex vivo induction of functional biomarkers to show how a patient’s live tumor cells respond to pathway stimulants (such as growth factors) and inhibitors (therapeutic drugs).

Building a Family
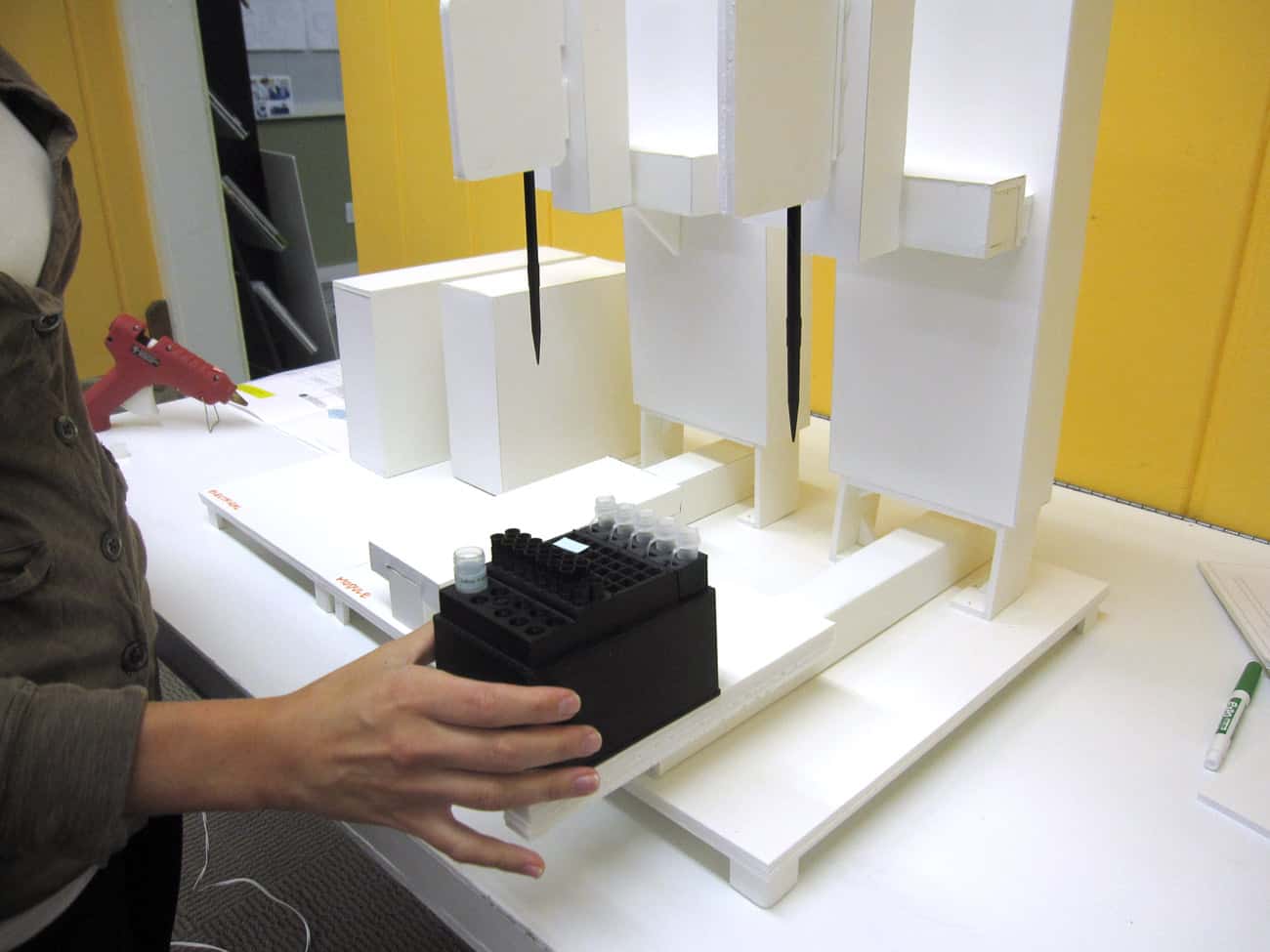
HSD began by observing the daily activity within radiology and pathology suites to understand the workflow, individual personnel involvement, patient processing, and workload. We investigated the current process for procuring and processing FNA/Core biopsies. By concentrating on the user experience and the needs of the environment, we were able to gain actionable feedback in regards to SnapPath® design opportunities. Spun out of leadership from Johns Hopkins, BioMarker Strategies developed a comprehensive development team including assay development, clinical research, systems and software design, and development. HSD worked early in the process to provide the foundation of the use workflow, providing contextual research regarding the process and protocols required for commercialization.

Building a Family
HSD began by observing the daily activity within radiology and pathology suites to understand the workflow, individual personnel involvement, patient processing, and workload. We investigated the current process for procuring and processing FNA/Core biopsies. By concentrating on the user experience and the needs of the environment, we were able to gain actionable feedback in regards to SnapPath® design opportunities. Spun out of leadership from Johns Hopkins, BioMarker Strategies developed a comprehensive development team including assay development, clinical research, systems and software design, and development. HSD worked early in the process to provide the foundation of the use workflow, providing contextual research regarding the process and protocols required for commercialization.

The Strategy | Our Process
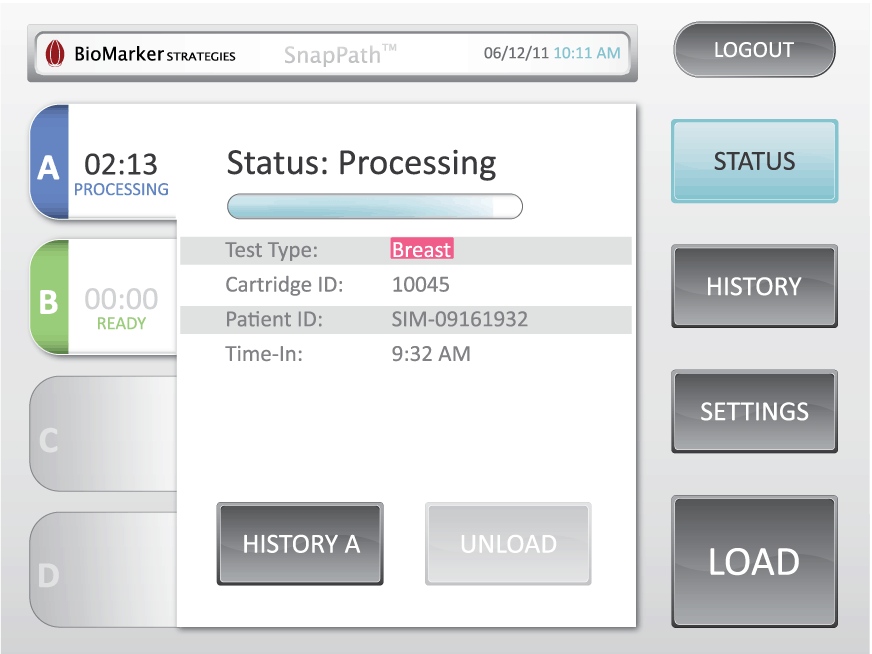
Understanding how biopsy samples travel throughout the current process enabled us to design a disposable cartridge that best fit that workflow. The complete cartridge design contains the test components eliminating the need for on-board fluidics and waste. Our role included design, engineering for manufacture, packaging, labeling, and system integration. As a key component of the system, the graphic user interface acts as the user’s method for operation. HSD began this process by developing the theory of operation, workflow, wireframe concepts, as well as methods to validate the concept. With the consideration of user feedback, the design was further developed to enhance the brand identity and perception to create the utmost experience to manage multiple processing cartridges.
The Strategy | Our Process
Understanding how biopsy samples travel throughout the current process enabled us to design a disposable cartridge that best fit that workflow. The complete cartridge design contains the test components eliminating the need for on-board fluidics and waste. Our role included design, engineering for manufacture, packaging, labeling, and system integration. As a key component of the system, the graphic user interface acts as the user’s method for operation. HSD began this process by developing the theory of operation, workflow, wireframe concepts, as well as methods to validate the concept. With the consideration of user feedback, the design was further developed to enhance the brand identity and perception to create the utmost experience to manage multiple processing cartridges.

Impact
The SnapPath® went under the De Novo regulatory classification for IVD devices. HSD partnered with Sparton Medical for the contract manufacturing. HSD assisted in design history files as well as Human Factor reports on the formative user studies to support the submission.

Impact
The SnapPath® went under the De Novo regulatory classification for IVD devices. HSD partnered with Sparton Medical for the contract manufacturing. HSD assisted in design history files as well as Human Factor reports on the formative user studies to support the submission.