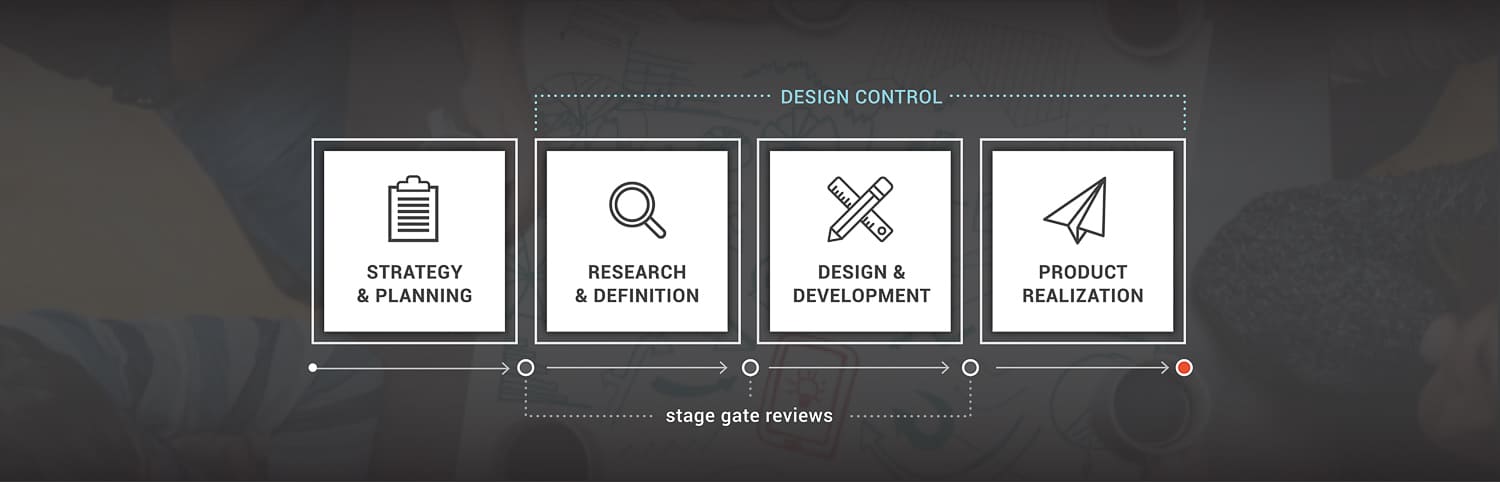
Our Process
Our phased approach provides a roadmap to
take new technology from idea to reality.
Business Strategy & Planning
- Project Plan
- Technology Assessment
- Documentation Assessment
- Project Planning
- Risk and Feasibility Planning
- Regulatory Path
Research & Definition
- Contextual Research
- Design Inputs and Requirements Definition
- Human Factors Requirements
- Initial Concept Ideation
Design & Product Development
Once we understand the goals, user needs, and product requirements, we begin the design and development phase. Our award-winning designers, in search of the most appropriate solutions, keep fluid creativity and innovation alive throughout concept ideation.
Typical activities in this phase include rapid sketching and rendering. These visual representations of concepts facilitate brainstorms, pin-ups, and design reviews. Sketches and models are also created to support ergonomic and aesthetic evaluations.
Our engineering team is involved early in the design process to help provide a proof of concept and guarantee design for manufacturability.
This phase may include:
- Proof of Concept
- System Architecture
- Design & Engineering
- Applied Human Factors
- Formative Usability Evaluations
- Design Inputs
Our user experience team plans and conducts formative studies early in the development process to identify potential issues and to support decision making throughout the project. Formative studies may be conducted multiple times during development to ensure the device meets user requirements and to ensure the best possible user interaction with the device. Formative studies can include heuristic evaluations, user interviews, or low-fidelity prototype testing. A final pre-validation formative evaluation with a working prototype is recommended for most medical devices since the results may predict the outcome of summative evaluation required in the validation phase.
Although we have already integrated CAD software tools in the development process, our Mechanical Engineers begin the generation of detailed parts and the database used for tooling and manufacturing in the development phase. Typically, we issue a final control database for use in the prototype and validation phase.
Realization and Manufacture
We have access to multiple manufacturing resources including in-house within the SteriPack Group itself, and can leverage this expertise specifically for our clients. In the Realization phase, we review pre-production parts against final specification requirements through a first article review process. For final quality assurance, it is typically necessary to update the database per specific manufacturing requirements. Once updated, the final database is transferred to manufacturing or directly to the client.
This phase may include:
- Design Transfer
- Manufacturing Liaison
- Production Ramp
- Engineering Support with Initial Product Launch