Design firm HS Design of Gladstone, New Jersey has again solidified itself as a true innovation
pioneer with two finalists in three categories for the 2019 Medical Design Excellence Awards
on June 11th in New York City.
The Medical Design Excellence Awards competition has become synonymous with innovation in the medical
design space. The MDEA competition accepts entries worldwide from companies and individuals involved in
the design, engineering, manufacturing, or distribution of finished medical devices.
This year, design firm HS Design of New Jersey has again solidified itself as a true innovation pioneer. With
two finalists being announced in three categories, HS Design is the only design firm this year with this
distinction.
In the category, “Digital Health Products and Mobile Medical Apps,” HSD partnered with the electroCore team
on the user research, industrial design, user interface and mechanical engineering. The design focused on
consumer healthcare parallels the novel technology features easy cleaning and simple intuitive operation.
The gammaCore SapphireTM, a non-invasive vagus nerve stimulator (nVNS), that treats pain related to migraine
and episodic cluster headache also is a finalist in the category “Over-the-Counter and Self-Care Products”.
Designed as a portable, easy-to-use technology, the therapy can be self-administered via placement on a
patient’s neck over the vagus nerve, stimulating the nerve’s fibers and resulting in a reduction of pain.
The MDEA competition has been designed to reward competitors who have really made strides in innovation
and design. It is open to finished medical devices, including instruments, machines, implants, in vitro reagents,
mobile medical applications, or other related products that are intended for the diagnosis, cure, mitigation,
treatment, or prevention of disease or other conditions in humans or animals.
“The MDEA awards are important to us, as they are not only judged by our peers, but also judged by practicing
medical experts, including doctors and nurses. Knowing that two of our programs reached the Finalist level is
humbling,” noted Tor Alden, Principal of HS Design, Inc. “The MDEA is a premier award and we’re thrilled to
be recognized by them in this way. The recognition means a tremendous amount to all the team members that
put so much passion in the development of both the gammaCore and SkinPen products. We are excited to see
them being recognized.”
In the MDEA Category for “Nonsurgical Hospital Supplies and Equipment,” HS Design is proud to announce
Bellus Medical SkinPen® Precision microneedling device as a Finalist this year for the event June 11th in New
York City. HS Design worked in conjunction with Bellus Medical and Sparton Medical Systems in achieving
this amazing announcement!
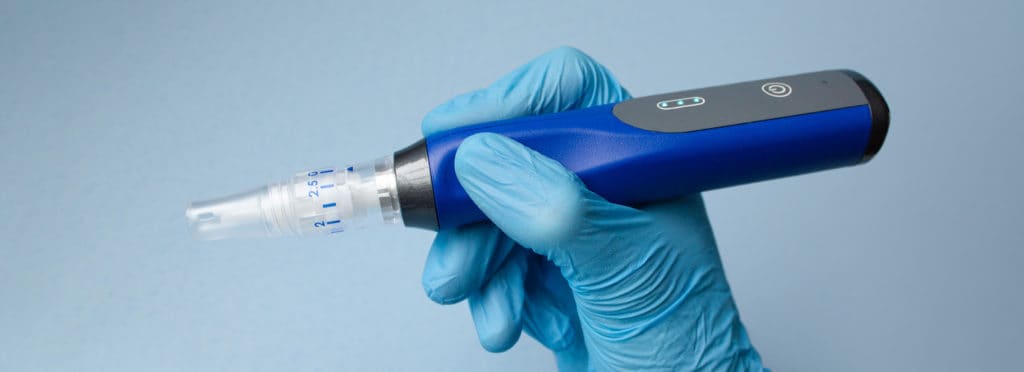
The Bellus SkinPen® Precision microneedling device new MDEA Finalist Status follows becoming the first
FDA-cleared microneedling device in 2018, that created a new class II category. Based on a series of
technological advances, the handheld device, non-invasively creates controlled micro-injuries clinically proven
to safely improve the appearance of facial acne scars for patients 22 years and older.
Joe Proctor, President of Bellus Medical went on to say, “Bellus Medical is honored to be recognized as MDEA
Finalist. Our goal was to create a gateway product to ensure patients’ safety as well as the comfort and usability
for the practitioner. SkinPen will continue to positively impact the lives of patients and their healthcare
providers who are committed to providing great results and patient safety.”
More fundamentally, SkinPen qualifies as “biocompatible” because none of its components pose an
unacceptable risk of a potential adverse biological response. Similarly, the device is compliant with the
Restriction of Hazardous Substances (RoHS), a European Union rule that restricts the use of hazardous
materials in electrical and electronic products.
HS Design’s human-centered product design approach combines researchers, human factors engineers,
designers, engineers, marketing and manufacturing experts to create a team specifically tailored to an individual
client’s needs. This process has helped create over 400 successful products in our 40-year history.
ABOUT electroCore, Inc.:
electroCore, Inc. is a commercial-stage bioelectronic medicine company dedicated to improving patient
outcomes through its platform non-invasive vagus nerve stimulation therapy initially focused on the treatment
of multiple conditions in neurology and rheumatology. The company’s initial targets are the preventative
treatment of cluster headache and acute treatment of migraine and episodic cluster headache. For more
information, visit www.electrocore.com.
ABOUT Bellus Medical:
Bellus Medical, the premier medical aesthetics company, is dedicated to helping leading aesthetic practices
around the world grow their businesses. We do that by delivering dramatic results in rejuvenation and restoration. Our non-invasive innovations – SkinPen®, the first FDA-cleared microneedling device; the post-
microneedling protocol SKINFUSE®; the light-activated cream Allumera®; and the platelet-rich plasma systems ProGenTM and RegenLab® – act as “gateway” products that draw new consumers to practices. Based in
Dallas, Texas, Bellus sets industry standards for efficacy, safety, and innovation. As a result, our customers
consistently deliver the best aesthetic care in the business. For more information, please visit
www.bellusmedical.com.
ABOUT HS Design:
HS Design (HSD) is FDA compliant and is an ISO 13485 certified user-centered design firm specializing in
research, experiential design and engineering for Medical, Scientific, consumer healthcare products and
delivery systems. HSD integrates insight, experience, and innovation with user needs and client core
competencies to develop products that exhibit a strategic advantage in the marketplace. The combination of
Research, HFE, Industrial Design, UI/UX, and Engineering allows HSD to design for diverse environments and
their implementation to manufacturing makes it a reality. HSD’s commitment to design excellence and strong
relationships with our client partners has yielded numerous Medical Device Excellence Awards.
HSD delivers innovative results through their unique ability to partner in development and combines creativity
with end users’ needs and manufacturing implementation. HS Design offers a variety of services specifically
aimed at aligning user needs to new ideas and technology to bring them to market quickly. For more
information, please visit https://hs-design.com/free-analysis/.
If you have any questions regarding information in these press releases please contact the company listed in the press release. Our complete disclaimer appears here.
Contact Information
Justin Starbird
The Aebli Group, LLC
http://aebligroup.com
(207) 939-2065
