Gladstone, NJ, September 15, 2019
HS Design, Inc. has been awarded a National Institutes of Health (NIH) National Cancer Institute Small Business Innovation Research (SBIR) Phase II contract under award number 75N91019C00039. This Phase II contract supports the research and development efforts of HS Design for developing Sympfiny® a novel dosing and dispensing system for multi-particulate cancer drugs initiated in a previous Phase I contract The Phase II funding is based on the results achieved in Phase I and the scientific and technical merit and commercial potential of the Phase II project proposed. Only Phase I awardees are eligible for a Phase II award.
Mr. Michael Quinn, VP of Design & Engineering and Principal Investigator at HSD stated, “The Phase II contract helps solve the chicken and egg problem: develop a device first or wait for the taste-masked multiparticulate formulations. Pharmaceutical companies are hesitant to work on medicinal formulas without a reliable delivery device. The Phase II contract is therefore vital to the continued development of the small form-factor Sympfiny system, also known as Sympfiny 1ml.”
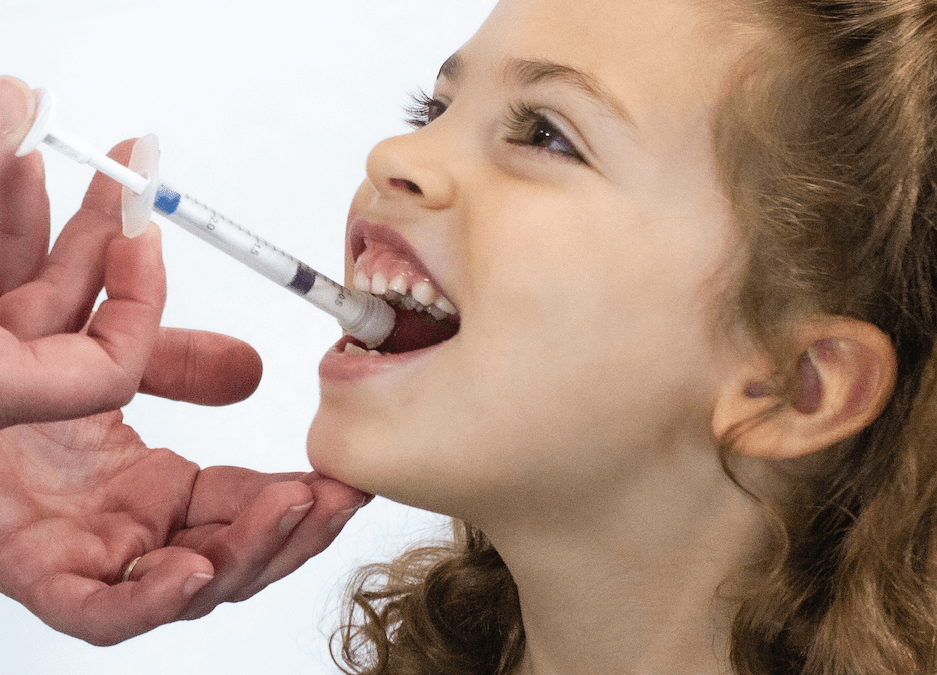
Pediatric cancer drugs are often dosed in very small volumes but also need to be adjusted based on body weight or a child’s size. The 1ml Sympfiny is ideally suited to this task. Sympfiny® is the first multiparticulate delivery device to dose the same as traditional liquid dispensers, creating an intuitive and user-friendly experience. Its proprietary dispensing technology is a revolutionary delivery system that enables a new generation of multiparticulate drug formulations to be delivered with high dose flexibility and with performance, features, and form factors that increase safe and effective administration of drugs.
The breakthrough system enables caregivers to store, extract, and deliver dry drugs with the same familiar technique used with liquid oral drugs. The patent-pending design allows a controlled and precise dosage and ensures all medication is dispensed directly to the child’s mouth. For more information on Sympfiny, please visit: https://sympfiny.com
“We are extremely grateful for the NIH’s continued support of our technology development,” noted Tor Alden, President of HS Design. The NIH is making it possible for us to contribute in commercializing a truly novel way for dispensing oral pediatric cancer medication. “
ABOUT HS Design:
HS Design, Inc. (HSD) is an award winning, user centered product development firm with a focus in the medical, healthcare, and life science marketplace. For over 40 years, HSD has been recognized for expertise in the areas of design research, strategy, human factors, user experience, industrial design, and mechanical engineering. HSD is a FDA compliant and ISO 13485 certified organization with team members proudly serving on the AAMI Human Factors Committee and assisting with Use and Usability guidance documents (ISO 62366 and IEC 62366). By approaching products from a user-centered perspective, HSD is confident in designing and developing products for a diverse environment. For more information about HSD, please visit: https://hs-design.com
About the SBIR/STTR:
The Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs, also known as America’s Seed Fund, are one of the largest sources of early-stage capital for technology commercialization in the United States. These programs allow US-owned and operated small businesses to engage in federal research and development that has a strong potential for commercialization. The purpose of these programs is to support small businesses with the most innovative, cutting-edge ideas that have the potential to become great commercial successes and make huge societal impacts.