In a recent episode of the HsDNA podcast, host Justin Starbird sat down with Tor Alden, Global VP/GM Design, Development, and Human Factors for HS Design, a Steripack company, to discuss the importance of choosing the right medical device product design firm for your startup, as well as “5 Traps Startups Need to Watch Out for When Engaging with a Product Design Firm“.
In this accompanying 5-part blog series, we’ll dive deeper into Tor’s top 5 traps, one by one, for a better understanding.
Trap 1: Planning & Understanding the Medical Product Development Process
Key points in this trap include:
Due diligence in selecting a partner, RFQ/RFP, Phase Methodology, Compliance, Regulatory Pathway, DHF/QMS, and IP Landscape
“Too often, I find that a startup will come to a design firm and get a generic project schedule and a generic quote, share it, get the funding needed, and then come back to the design firm and not understand that it’s a long-term relationship, almost like a marriage with the developing partners,” explains Tor.
One major trap a medical device startup can fall into is needing to understand and plan for the entire development process thoroughly. Therefore, when it comes to product development for a medical device, it starts very early with strategy, planning, and understanding.
Have you secured your intellectual property (IP)? Do you fully understand the regulatory process and where your product fits within it (510k, DeNovo, PMA)? Have you addressed the marketing aspects, such as if your product works within current Current Procedural Terminology (CPT) codes? Finally, does your current development plan address future quality assurance (QA) and good manufacturing practices (GMP)? The startup should address all of these concerns before the start of the project.
Understanding your needs, your journey, what it includes, and where you are on it can help you determine which firm has the knowledge, experience, capabilities, and services to complement your startup and lead you to success.
As no two startups are the same, no two medical device design firms are.
For example, HSD’s services range from strategy and planning to market realization, including research, human factors, UX/UI Design, industrial design, engineering technology, and prototyping, making HSD a collaborative team to complement client needs.
“Key to this,” Tor notes, “is developing a meaningful product requirement specification (PRS) and developing that into a formal Request for Proposal (RFP) that will allow you to compare apples with apples as opposed to apples with oranges with a product development firm.”
Understanding the medical device development process and your product requirements can help you choose the firm that best suits your needs, leading to a better successful outcome.
Stage gate processes, such as HSD’s phased approach, are the most common development models in the medical device industry and provide a roadmap to take new technology from idea to reality. Throughout the process, regulatory requirements are taken into consideration and have a significant impact on the activities and decisions made.
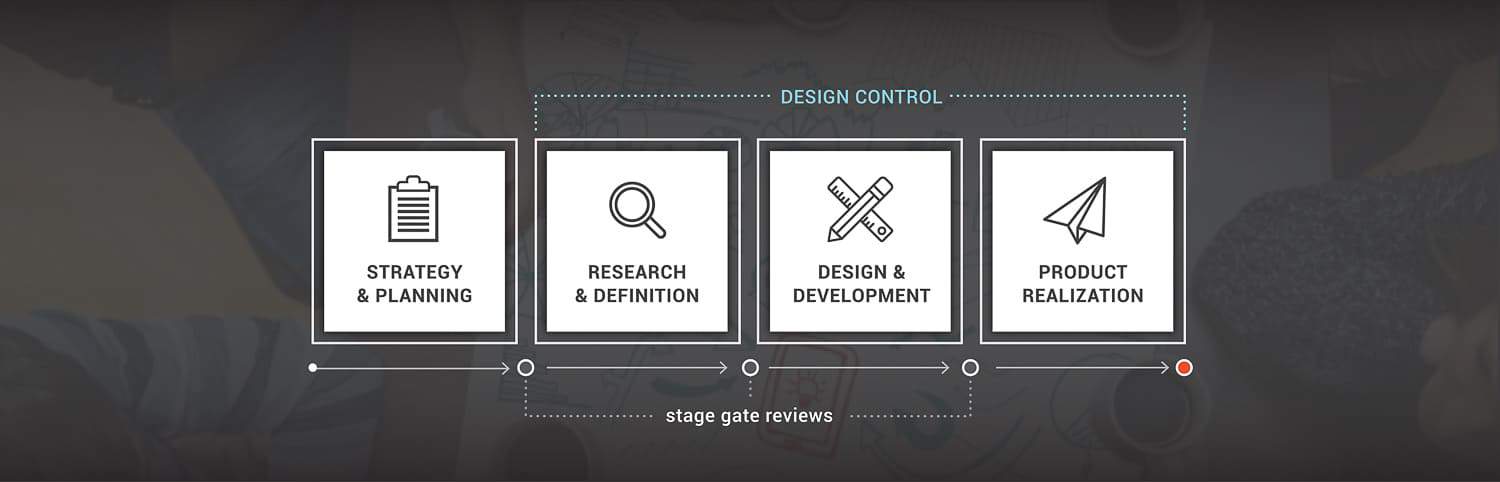

Considerations throughout this process include:
Compliance – What compliance does your product need to follow?
- W21CFR 820 – Regulation/Medical Device Good Manufacturing Practices
- IEC 61010 – Standard for Measurement, Control and Laboratory Equipment
- IEC 60601 – Medical Electrical Equipment – General requirements for basic safety and essential performance
- IEC 62304 – Software Development Life Cycle
- IEC 62366 – the international standard that covers the application of usability engineering to medical devices
- ISO 14971 – Application of risk management to medical devices
The Regulatory Pathway
- US Food & Drug Administration (FDA), European Medicine Agency (EMA), and Conformite Europeenne (CE) regulations
- Understanding the FDA Waterfall regarding Verification and Validation
- If you intend to sell globally, you need to address both FDA, EMA & CE.
- Reimbursement Strategies
- Do you have a reimbursement & marketing strategy? CPT codes are critical to attract VC firms when investing.
- Risk Assessment and Management
- De-risking your product along the way with Design History Files (DHF) and a Quality Management System (QMS)?
- According to FDA 21 CFR 820.30, a Design History File (DHF) is a compilation of records that carries the design history of a finished medical device. The DHF includes all the necessary records to demonstrate that the device design was developed as per the approved design plan and requirements.
YOUR DHF INCLUDES:
- Design and development planning – design plan document, developed according to this part.
- Design input – procedures for establishing design input that addresses the intended use and user needs, and the approved design input documentation itself.
- Design output – procedure for defining and documenting design output in compliance with this part and the approved design output documentation itself.
- Design review – procedure for conducting reviews of your design process and any documentation associated with the reviews.
- Design verification – a document describing your design validation process and the approved results of the design validation.
- Design validation – specific procedure and testing conditions used for design validation, as well as the approved results of the design validation process.
- Design transfer – documented product specifications that are developed in compliance with this part and a description of the process used.
- Design changes – documented design change processes and documentation pertaining to any design changes that have taken place.
IP Landscape
Intellectual property is a competitive advantage when creating value for your startup, however, it is only as strong as someone being able to enforce it.
Be sure to consider:
- Provisional – earliest form of IP protection gives a one year window of protection from the US Patent & Tradmark Office (USPTO)
- Freedom to Operate (FTO) Analysis
- UTILITY vs DESIGN Patent applications. Utility offer more value, but Design should be considered if the design in highly distinctive.
- International protection – you must protect your IP by filing for a patent in a specific country, but to file overseas patent, you myct already have a USPTO license.
To conclude Trap 1, host Justin Starbird points out, “One of the more fascinating pieces that you brought up and that people may not realize is that picking the right partner is really like a marriage. You’re going to have things that go well at the beginning. It’s going to feel great. But you’re going to hit some bumps in the road or maybe challenges along the way that you weren’t expecting. You need to overcome those and be able to listen to not just the advice but then heed it and follow through on what needs to happen. I think developing relationships in a way where everybody feels confident about where the project is going is a great start.”
“That’s a perfect segue into what I would consider Trap 2,” Tor points out.