Introducing Sympfiny™
A Next Generation Multi-Particulate Delivery System
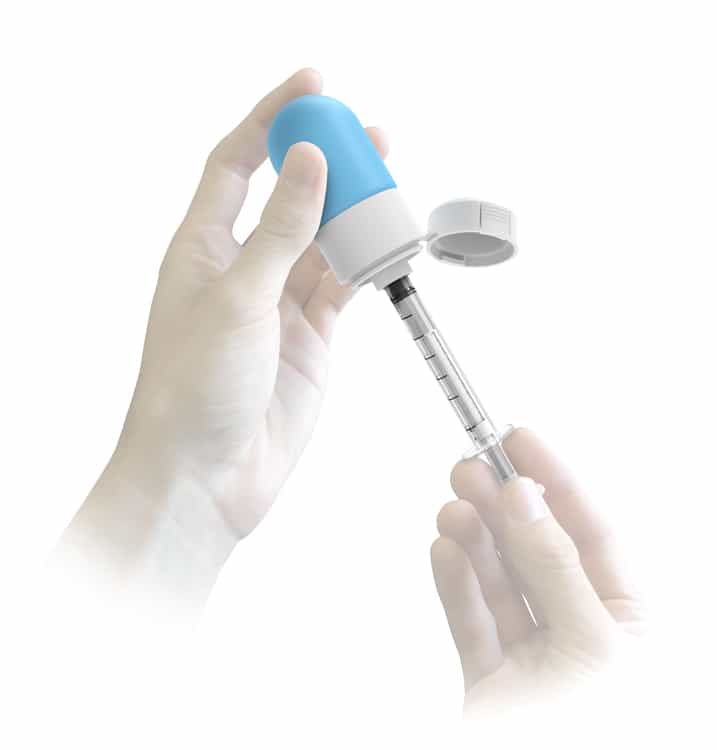
Sympfiny™ is an innovative system for dosing and dispensing multiparticulate, dry powder, and microsphere, drug formulations for oral delivery.
Intuitively Simple & Child Friendly
Sympfiny™ is the first multiparticulate delivery device to dose the same as traditional liquid dispensers, creating an intuitive and user friendly experience.
The breakthrough system designed by HSD Ventures™ enables caregivers to store, extract, and deliver dry drugs with the same familiar technique used with liquid oral drugs.
The patent pending design allows controlled and precise dosage and ensures all medication is dispensed directly to the child’s mouth.
Designed with the entire supply chain in mind, the system fits common bottle sizes and can be pre-filled or filled at a pharmacy. The internal valve and bottle cap protects the drug through storage and use. The re-usable syringe comes in two sizes and has selectable dose settings.
Why Multiparticulates?
Oral Multiparticulates provide an excellent platform for pediatric medicines.
- No inherent taste so it’s the ideal substrate for taste masking, increasing compliance and reducing possible rejection by child
- Allows multitude of dose flexibility
- Reduces and possibly eliminates need for preservatives, sweeteners, flavorants, dyes, etc.
- May be dosed with or without water
- The technology can be applied to the active ingredients of a wide range of drug molecules.
- Different drugs formulated as MP powders can be combined to form homogenous mixtures, allowing the administration of multiple drugs and potentially multiple different drug release profiles.
- MP powder flows similar to a liquid and ideally with a non-gritty mouth feel.
- MP powders are typically stable across all global zones and therefore do not require cold chain storage.
Almost three million children die needlessly each year from pneumonia and diarrhea alone.
The treatment of choice for pneumonia – amoxicillin – appears to be available in developing countries, however critically, not in the recommended dose and formulation.
What are Multiparticulates?
Oral multiparticulate technology, in the form of beads, mini-tablets and microspheres with coated and/or matrix architecture, offers a wide range of drug release profile flexibility for single or multiple drug combinations. They can be formulated as modified-release (e.g. extended, delayed, pulsed), immediate-release, bioavailability-enhanced, or taste-masked dosage forms.
In addition, multiparticulates provide predictable and consistent gastrointestinal transit and lower chances of undesirable events (e.g., dose dumping, colonic streaming) associated with tablets. These multiparticulates can be dosed within capsules, tablets (microspheres, coated beads) or sachets.
Pfizer has developed a multiparticulate (MP) pediatric formulation technology that addresses taste, storage, and other factors for safe, accurate and adherent administration of medicines to children in low resource global health settings. This technology platform is one which Pfizer has been openly sharing with others to encourage the greater implementation of the approach.
Sympfiny™ features:
Versatile Bulk Container
- On site fill capability
- Engineered to be humidity resistant
- Customizable for your volume and branding needs
- Compatible with child-proof caps
Intuitive Dispensing Platform
- Dispenses similarly to liquid oral syringe
- One handed operation
- Reusable and easy to clean
Child Friendly Delivery System
- Spill-proof
- Direct delivery to child’s mouth
- Chew resistant outer shell
- Clog resistant
Novel Interlocking Design
- Simple to use
- Prevents accidental drug spills
- Automatically seals against contaminants
User Friendly Dose Settings
- Multiple dose settings in a single platform
- Customizable by pharmacist or caregiver to meet the needs of the patient population

Download the Sympfiny™ Spec Sheet
Press Release: Institute for Pediatric Innovation and Pfizer collaborated on the open innovation pediatric device challenge.
The Institute for Pediatric Innovation (IPI) recently collaborated with Pfizer in a historic opportunity to branch out into pediatric global health. Pfizer has developed a state-of- the-art solid multiparticulate (MP) drug reformulation technology. This technology addresses taste, storage, and other factors essential for safe, accurate, and adherent administration, making it ideal for administering medicines to children in low-resource settings.
IPI and Pfizer partnered in an open innovation challenge to solicit and support innovative ideas for a system consisting of a package and dispensing device that will be used to deliver oral solid MP-formulated medicines to children. A panel led by IPI composed of engineers, end users, and experts from the Gates Foundation, New England Pediatric Device Consortium, PATH, and Pfizer, reviewed submissions to decide which proposals would receive funding.
Proposals were evaluated on four main criteria: dose accuracy, cost, end user ease of use, and cultural appropriateness. Pfizer offered up to $50,000 to the winner. The challenge attracted 25 submissions. The panel awarded $50,000 grants to two applicants.
HSD’s novel design features a proprietary bottle for storage of the multiparticulates and a syringe for dosing. This novel system is designed to be used similar to existing liquid oral syringes and vials, minimizing the length of the learning curve. HSD has partnered with The Röchling Group; a global contract manufacturer specializing in the manufacture of drugs delivery devices, to fully develop the delivery system.
About HSD Ventures™
HSD Ventures™ specializes in developing partnerships for the design and development of next generation medical devices and pharmaceutical delivery systems. HSD Ventures is part of HS Design (HSD), an ISO 13485 certified product development firm with a focus on medical, healthcare, and life science markets. HSD continues to be recognized for nearly 50 years as experts in product development for Fortune 100 and start-up companies.