Search for a cure
Medical + Life Sciences Case Study

Biomarker Strategies SnapPath™
SnapPath™ is an innovative IVD instrument for live cell processing. HS Design’s expertise was involved in Design Research, Industrial Design, Systems Engineering, User interface design, Mechanical engineering, prototyping and ultimately formative verification and submitted to the FDA under the 510k de novo process.
The evolution of “personalized medicine” is changing the way oncologists diagnose and treat cancer

Brand
The evolution of “personalized medicine” is changing the way oncologists diagnose and treat cancer. Over the past few years, there has been a clear shift in the oncology pipeline away from traditional cytotoxic drugs to more molecularly targeted agents. Similarly, there has been a shift away from simple, single-target drugs towards inhibition of complex signalingpathways. In each case, the need for more complex functional biomarkers has emerged. SnapPath® not only provides a means for preserving functional information within a cell, it more importantly enables the generation of PathMap™ profiles.
Product
The SnapPath® biomarker testing system is an automated live tumor cell processing platform that enables next-generation predictive tests — known as PathMap™ profiles — to guide targeted drug therapy selection for individual cancer patients.The team sought the input of direct Users to interpret their perceptions of the SnapPath™ platform and the possible integration into their lab environment or workflow.

Creating An Experience
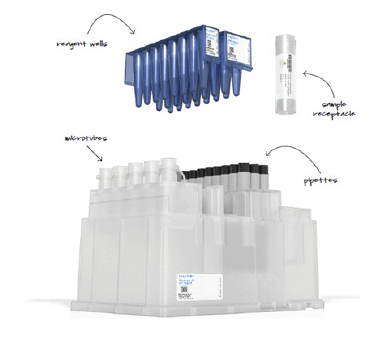
SnapPath™ is a system that comprises an Instrument and a Cartridge. The Instrument is to be a Pathology-based device that is fully integrated and interfaces directly with a Cartridge preloaded with reagents and disposables. The platform processes I’ve biopsy specimens procured from FNA procedures but has potential to include surgical and core sampleds to date. molecular testing of solid tumors has relied primarily on the testing of dead. formalin-fixed samples. The SnapPath® platform uniquely enables the ex vivo induction of functional biomarkers to show how a patient’s live tumor cells respond to pathway sti mulants (such as growth factors) and inhibitors (therapeutic drugs).
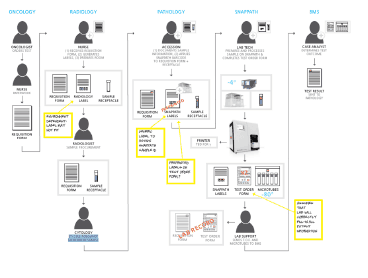
Site visits included top rated institutions such at U Penn, Jefferson, Johns Hopkins, and MD Anderson. From this work, HSD synthesized the data to integrate a process
including oncology, pathology and radiology.
Building a Family
HSD began by observing the daily activity within radiology and pathology suites to understand the
workflow, individual personnel involvement, patient processing. and workload. We investigated the current process for procuring and processing FNA/Core biopsies. By concentrating on the user experience and the needs of the environment, we were able to gain actionable feedback in regards to SnapPath design opportunities. Spun out of leadership from Johns Hopkins. BioMarker Strategies developed a comprehensive development team including assay development, clinical research, systems and software design, and development. HSD worked early in the process to provide the foundation of the use workflow, providing contextual research regarding the process and protocols required for commercialization.
The Strategy | Our Process
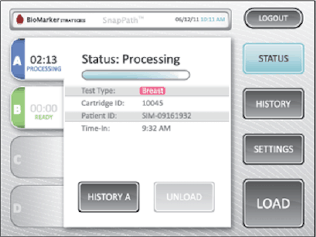
By understanding how biopsy samples travel throughout the current process enabled us to design a disposable cartridge that best fit that workflow. The complete cartridge design contains the test components eliminati ng the need for on-board fluid ics and waste. Our role included design, engineering for manufacture, packaging, labeling, and system integration. As a key component of the system. the graphic user interface, acts as the user’s method for operation. HSD began this process by developing the theory of operation, workflow, wireframe concepts, as well as methods to validate the concept. With the consideration of user feed back, the design was further developed to enhance the brand identity and perception t o create the utmost experience to manage multiple processing cartridges.
We discovered that our client needed flexibility in the cartridge design
to adapt to future iterations of their test.
Impact
The SnapPath went under the De Novo regulatory classification for IVD devices. HSD partnered with
Sparton Medical for the contract manufacturing. HSD assisted in design history files as well as Human
Factor reports o n the formative user studies to support the submission.
